Phone: +62-21 22116225 | Information@seameo-recfon.org
Biochemical Assessment
Biochemical Assessment
What is biochemical assessment?
Biochemical assessment is a laboratory-based evaluation that measures nutrient levels, metabolites, and key biochemical markers in biological samples.
Why is biochemical assessment important?
Biochemical assessment delivers reliable, quantitative data that cannot be obtained through observation alone. These results support the detection of nutrient deficiencies or excesses, guide clinical and nutritional interventions, and generate evidence that contributes to better health planning and public nutrition policies. Our laboratory ensures high-quality testing to support informed decision-making for both individuals and programs.
Our Services:
Retinol (Vitamin A)
Description
Retinol is the main circulating form of vitamin A, released from the liver with retinol-binding protein and essential for vision, epithelial integrity, and gene regulation. Serum retinol reflects liver vitamin A stores only when they are very low (<0.07 µmol/g liver) or very high (>1.05 µmol/g liver); within the normal range, concentrations are homeostatically regulated and may not indicate dietary intake or early deficiency in individuals.
At the population level, the distribution of serum retinol values, and the proportion falling below established cut-offs, can help identify whether vitamin A deficiency is a public health concern. Testing is commonly conducted among young children, who are most vulnerable. Vitamin A deficiency increases susceptibility to infections, raises childhood mortality risk, and remains the leading preventable cause of childhood blindness worldwide.
Useful for
- Assessing vitamin A status at the population level
- Identifying groups at risk of vitamin A deficiency
- Supporting micronutrient surveillance and nutrition program monitoring
- Evaluating public health interventions targeting vitamin A deficiency
Method
High Preasure Liquid Cromatography (HPLC) with UV detection (MU.7.2.3)
Lower Limit of Quantitation (LLOQ): 0.293 µmol/L
Sample Requirements
Sample type:
- Serum (preferred)
- Plasma (heparin)
Patient preparation:
- Overnight fasting (12–14 hours)
- No alcohol consumption 24 hours before collection
Sample volume: 200 µL
Sample Handling and Preparation
- Use light-protected (amber/dark) serum containers for submission.
- Hemolyzed, lipemic, or repeatedly thawed samples are not accepted.
Sample stability:
- Refrigerate whole blood immediately and centrifuge within a few days.
- When protected from light, serum vitamin A is stable for:
— up to 1 week at 4 °C
— at least 1 year at –20 °C
— at least 16 months at –70 °C
Quality Control (QC)
- National Institute of Standards and Technology (NIST) SRM 968f
- Pooled serum controls
Reference Values
Deficient: < 0.70 µmol/L
Low: < 1.05 µmol/L
Acceptable: ≥ 1.05 µmol/L
Turnaround Time
Results are available within 14 working days for up to 160 samples. For larger batches, please contact the laboratory team.
References
- Petunjuk Permohonan Pemeriksaan Contoh Uji (F.7.4.8)
- CDC, WHO, Nutrition International, UNICEF. Micronutrient Survey Manual. WHO, 2020.
- Tanumihardjo SA et al. BOND Vitamin A Review. J Nutr. 2016;146:1816S–48S.
- WHO. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. 2011.
*) Accredited by KAN 17025:2017
For more information please contact:
lab@seameo-recfon.org
α-Tocopherol (Vitamin E)
Description
Vitamin E contributes to the normal maintenance of biomembranes the vascular system, and the nervous system, and provides antioxidant protection for vitamin A. The level of vitamin E in the plasma or serum after a 12- to 14-hour fast reflects the individual’s reserve status.
Currently, the understanding of the specific actions of vitamin E is very incomplete. The tocopherols (vitamin E and related fat-soluble compounds) function as antioxidants and free-radical scavengers, protecting the integrity of unsaturated lipids in the biomembranes of all cells and preserving retinol from oxidative destruction. Vitamin E is known to promote the formation of prostacyclin in endothelial cells and to inhibit the formation of thromboxanes in thrombocytes, thereby minimizing the aggregation of thrombocytes at the surface of the endothelium. Those influences on thrombocyte aggregation may be of significance in relation to risks for coronary atherosclerosis and thrombosis.
Useful for
- Detecting vitamin E deficiency
- Evaluating individuals with neuromuscular symptoms or suspected oxidative stress
- Monitoring response to vitamin E supplementation
- Assessing vitamin E status in conditions with fat malabsorption
Method
High-Performance Liquid Chromatography (HPLC) with UV detection (MU.7.2.4)
Limit of Quantitation (LOQ): 7.163 µmol/L
Sample Requirements
Sample type:
- Serum
- Plasma (heparin)
Patient preparation:
- Overnight fasting (12–14 hours)
- No alcohol consumption within 24 hours before collection
Sample volume: 200 µL
Sample Handling and Preparation
- Submit samples in light-protected (amber/dark) serum containers.
- Hemolyzed, lipemic, or repeatedly thawed samples are not accepted.
Sample stability:
- Whole blood should be refrigerated immediately and centrifuged within a few days.
- When protected from light, serum vitamin E is stable for:
— up to 1 week at 4 °C
— at least 1 year at –20 °C
— at least 16 months at –70 °C
Quality Control (QC)
- National Institute of Standards and Technology (NIST) SRM 968f
- Pooled serum controls
Reference Values
Deficient: < 11.6 µmol/L
Low: 11.6–16.2 µmol/L
Acceptable: ≥ 16.2 µmol/L
Turnaround Time
Results are available within 14 working days for up to 160 samples. For larger batches, please contact the laboratory team.
References
- Petunjuk Permohonan Pemeriksaan Contoh Uji (F.7.4.8)
- Sauberlich HE. Laboratory Tests for the Assessment of Nutritional Status. 2nd ed. Routledge; 1999.
*) Accredited by KAN 17025:2017
For more information please contact:
lab@seameo-recfon.org
β-Carotene
Description
β-Carotene is a provitamin A carotenoid and an essential dietary nutrient found in many fruits and vegetables, contributing to their characteristic orange pigmentation. In the body, excess β-carotene is stored mainly in adipose tissue. High intake may lead to carotenodermia, a harmless condition characterized by orange discoloration of the skin. β-Carotene deficiency can occur in individuals with poor dietary intake or conditions that impair fat absorption, including cystic fibrosis, chronic pancreatic disorders (such as pancreatitis or pancreatic insufficiency), and malabsorption syndromes such as celiac disease.
Useful for
- Detecting deficiency or excess of β-carotene
- Monitoring β-carotene supplementation or dietary intake
- Supporting assessment of vitamin A precursor status
- Evaluating malabsorption disorders affecting fat-soluble nutrients
Method
High-Performance Liquid Chromatography (HPLC)
Limit of Quantitation (LOQ): 0.133 µmol/L
Sample Requirements
Sample type:
- Serum
- Plasma (heparin)
Patient preparation:
- Overnight fasting (12–14 hours)
- No alcohol intake within 24 hours before collection
Sample volume:
- Serum/Plasma: 200 µL
Sample Handling and Preparation
- Submit samples in light-protected (amber/dark) serum containers.
- Hemolyzed, lipemic, or repeatedly thawed samples are not accepted.
Sample stability:
- Whole blood should be refrigerated immediately and centrifuged within a few days.
- When protected from light, β-carotene is stable for:
— up to 1 week at 4 °C
— at least 1 year at –20 °C
— at least 16 months at –70 °C
Quality Control (QC)
- National Institute of Standards and Technology (NIST) SRM 968f
- Pooled serum controls
Reference Values
Normal: 0.3–0.6 µmol/L
Turnaround Time
Results are available within 14 working days for up to 160 samples. For larger batches, please contact the laboratory team.
References
- Petunjuk Permohonan Pemeriksaan Contoh Uji (F.7.4.8)
- CDC, WHO, Nutrition International, UNICEF. Micronutrient Survey Manual. WHO, 2020.
- Sauberlich HE. Laboratory Tests for the Assessment of Nutritional Status. 2nd ed. Routledge; 1999.
*) Accredited by KAN 17025:2017
For more information please contact:
lab@seameo-recfon.org
Zinc*
Description
Serum zinc is the primary biochemical indicator for assessing zinc deficiency at the population level. It reflects dietary zinc intake and responds consistently to zinc supplementation, making it useful for identifying populations or subgroups at higher risk of deficiency. Although reference data exist for most age and sex groups, serum zinc cannot be used to diagnose or treat zinc deficiency in individuals, as circulating zinc does not directly represent total body zinc status.
Serum zinc concentrations are influenced by multiple factors, including recent meals, time of day, age, sex, hormonal contraceptive use, inflammation, and systemic infections. Because inflammation can depress serum zinc levels, the International Zinc Nutrition Consultative Group (IZiNCG) recommends measuring inflammatory markers together with zinc and applying statistical adjustments when significant negative correlations are observed.
Useful for
- Assessing population-level zinc deficiency
- Monitoring response to zinc supplementation in surveys or research
- Identifying at-risk groups in nutrition programs
- Supporting studies related to growth, immune function, and inflammation
Method
Atomic Absorption Spectrophotometry/AAS (MU.7.2.2)
Lower Limit of Quantitation (LLOQ): 4.03 µmol/L
Upper Limit of Quantitation (ULOQ): 60.63 µmol/L
Sample Requirements
Sample type:
- Serum
- Plasma (heparin)
Patient preparation:
- Fasting for at least 8 hours is recommended.
- Specimens should be collected at a consistent time of day.
- The venipuncture site should be clean and free from powder or lotion.
Sample volume:
- Serum/Plasma: 600 µL
Sample Handling and Preparation
To minimize zinc contamination:
- Use zinc-free needles and trace-metal–free blood collection tubes.
- Process samples in a dust-free, CO₂-free environment.
- Phlebotomists must wear powder-free gloves.
- Use acid-washed serum cups and pipette tips.
Sample stability:
- Refrigerate whole blood and separate serum within 1–2 days.
- Serum zinc is stable for:
— 1 week at 4 °C
— 1 year at –20 °C
Quality Control (QC)
- Randox Clinical Chemistry Control Serum
Reference Values
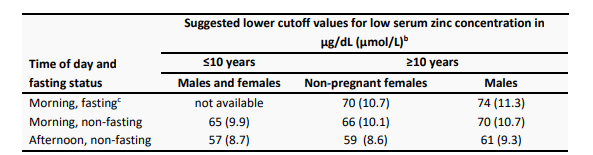
Turnaround Time
Results are available within 14 working days for up to 160 samples. For larger batches, please contact the laboratory team.
References
- Petunjuk Permohonan Pemeriksaan Contoh Uji (F.7.4.8)
- CDC, WHO, Nutrition International, UNICEF. Micronutrient Survey Manual. WHO, 2020.
- King JC et al. Biomarkers of Nutrition for Development (BOND) – Zinc Review. J Nutr. 2016;146:858S–885S.
*) Accredited by KAN 17025:2017
For more information please contact:
lab@seameo-recfon.org
Vitamin D-25-OH
Description
Vitamin D is essential for proper bone and tooth formation and for maintaining calcium, phosphorus, and magnesium balance. In the blood, 25-hydroxyvitamin D [25(OH)D] is the major circulating form and the best biomarker for assessing vitamin D status because it is abundant, stable, and has a long half-life. It serves as the precursor to the biologically active hormone, 1,25-dihydroxyvitamin D. Vitamin D is obtained from sunlight (vitamin D3) and diet or supplements (vitamin D2 and D3). Both forms are converted in the liver and kidneys into the active hormone. Some assays measure total 25(OH)D, while others separately quantify D2 and D3.
Vitamin D deficiency leads to impaired mineralization, causing rickets in children and osteomalacia in adults. Beyond bone health, vitamin D influences immune function, cell growth, and may play roles in autoimmunity and cancer. Individuals at higher risk of deficiency include older adults, people with limited sun exposure, darker skin pigmentation, obesity, or chronic medication use.
Useful for
- Detecting vitamin D deficiency or insufficiency
- Monitoring adequacy of vitamin D supplementation
- Assessing risk of bone health disorders (rickets, osteomalacia)
- Supporting evaluations of immune function and chronic disease risk
Method
Enzyme-Linked Immunosorbent Assay/ ELISA (MU.7.2.5)
Lower Limit of Quantitation (LLOQ): 3.95 ng/mL
Upper Limit of Quantitation (ULOQ): 116.78 ng/mL
Sample Requirements
Sample type:
- Serum
Patient preparation:
- Overnight fasting (12–14 hours)
Sample volume:
- Serum: 50 µL
Sample Handling and Preparation
- Hemolyzed, lipemic, or repeatedly thawed samples are not accepted.
- Do not use EDTA plasma.
Sample stability:
- 25(OH)D is highly stable. Whole blood processing may be delayed up to 2 days.
- Serum is stable for:
— 2 weeks at 4 °C
— ≥1 year at –20 °C
Quality Control (QC)
- NIST SRM 972a
- Internal quality controls (manufacturer kit controls)
- Pooled serum controls
Reference Values
The Endocrine Society, a global organization representing professionals from the field of endocrinology, defines vitamin D deficiency as 25(OH)D concentrations below 50 nmol/L 20 ng/mL) and vitamin D insufficiency as 52.0–72.5 nmol/L (21–29 ng/mL), based on multiple health outcomes, including but not limited to musculoskeletal outcomes. The European Food Safety Authority (EFSA) has suggested similar cutoff values.
References
- Petunjuk Permohonan Pemeriksaan Contoh Uji (F.7.4.8)
- CDC, WHO, Nutrition International, UNICEF. Micronutrient Survey Manual. WHO, 2020.
*) Accredited by KAN 17025:2017
For more information please contact:
lab@seameo-recfon.org
7-plex Human Micronutrients
Description
The Q-Plex Human Micronutrient v2 (7-Plex) is a multiplex chemiluminescent immunoassay designed for efficient assessment of micronutrient status in population-level surveillance. Micronutrient deficiencies disproportionately affect children and women of reproductive age, especially in low- and middle-income countries, and contribute to increased morbidity, mortality, and impaired developmental outcomes. This multiplex assay enables simultaneous measurement of seven key biomarkers—AGP, CRP, Ferritin, HRP2, RBP4, sTfR, and Thyroglobulin—in a single, low-volume sample, supporting large-scale surveys and program monitoring. Assay performance has been verified and calibrated with WHO/NIBSC international standards which improves confidence in data accuracy and reduces inter-laboratory bias.
Useful For
- Population-level micronutrient surveillance
- Identification of populations at risk of micronutrient deficiencies
- Monitoring nutrition program implementation and impact
- Efficient measurement of inflammation markers and micronutrient status in large surveys
Method
Multiplex chemiluminescent immunoassay
| Analyte | Upper Limit if Quantitation (ULOQ) | Lower Limit of Quantitation (LLOQ) |
|---|---|---|
| AGP | 16.8 g/L | 0.02 g/L |
| CRP | 44.8 mg/L | 0.00384 mg/L |
| Ferritin | 1042.0 µg/L | 0.92 µg/L |
| sTfR | 152.8 mg/L | 0.168 mg/L |
| RBP4 | 42 µmol/L | 0.048 µmol/L |
| Thyroglobulin | 735.2 µg/L | 0.6 µg/L |
| HRP2 | 68 µg/L | 0.072 µg/L |
Sample Requirements
Sample type:
- Serum (preferred)
- Plasma (heparin)
Sample volume:
- 25 µL
Sample Handling and Preparation
- Avoid haemolyzed, lipemic, or repeatedly thawed samples.
- Follow standard serum/plasma separation procedures.
- Store and ship samples according to stability requirements of the analytes
- Maintain frozen conditions for long-distance survey shipments.
Quality Control (QC)
- Calibration aligned with WHO/NIBSC standards
- Participation in CDC Performance Verification Program for Serum Micronutrients
- Internal kit controls for each assay plate
Reference Values
Reference intervals depend on each analyte and should be interpreted using WHO, BRINDA, or survey-validated cut-offs.
Turnaround Time
Results are available within 14 working days for up to 150 samples. For larger batches, please contact the laboratory team.
References
- Petunjuk Permohonan Pemeriksaan Contoh Uji (F.7.4.8)
- CDC, WHO, Nutrition International, UNICEF. Micronutrient Survey Manual. WHO, 2020.
For more information please contact:
lab@seameo-recfon.org
11-plex Environmental Enteric Dysfunction
Description
Environmental Enteric Dysfunction (EED) is characterized by chronic intestinal inflammation, impaired barrier integrity, and reduced nutrient absorption. It is common in settings with high enteric pathogen exposure and inadequate sanitation. EED is associated with adverse outcomes including stunted growth, impaired cognitive development, reduced vaccine responsiveness, and increased susceptibility to infections such as pneumonia, acute diarrhea, and malaria.
The Q-Plex Human Environmental Enteric Dysfunction (11-Plex) assay provides a multiplex chemiluminescent platform to measure a comprehensive profile of biomarkers related to intestinal function, inflammation, nutrition, metabolic regulation, growth, and malaria exposure. The platform enables efficient, low-volume assessment suitable for population surveillance and research applications.
Useful For
- Population-level assessment of environmental enteric dysfunction
- Profiling inflammatory, nutritional, metabolic, and intestinal biomarkers
- Identifying subgroups at higher risk for EED-related complications
- Supporting intervention programs and research studies
Method
Multiplex chemiluminescent immunoassay
| Analyte | Upper Limit if Quantitation (ULOQ) | Lower Limit of Quantitation (LLOQ) |
|---|---|---|
| AGP | 2.7 g/L | 0.0041 g/L |
| CRP | 57.8 mg/L | 0.23 mg/L |
| Ferritin | 1284.4 µg/L | 1.3 µg/L |
| sTfR | 755.2 mg/L | 1.0 mg/L |
| RBP4 | 17.9 µmol/L | 0.025 µmol/L |
| Thyroglobulin | 128.0 µg/L | 0.18 µg/L |
| HRP2 | 9.7 µg/L | 0.036 µg/L |
| I-FABP | 21,526 pg/mL | 23.7 pg/mL |
| sCD14 | 25,352 ng/mL | 29.8 ng/mL |
| IGF-1 | 2,033.7 ng/mL | 3.2 ng/mL |
| FGF21 | 6,977.9 Pg/mL | 9.6 Pg/mL |
Sample Requirements
Sample type:
- Serum (preferred)
- Plasma (heparin)
Sample volume:
- 25 µL
Sample Handling and Preparation
- Avoid haemolyzed, lipemic, or repeatedly thawed samples.
- Follow standard serum/plasma separation procedures.
- Store and ship samples according to stability requirements of the analytes
- Maintain frozen conditions for long-distance survey shipments.
Quality Control (QC)
- Calibration aligned with WHO/NIBSC standards
- Participation in CDC Performance Verification Program for Serum Micronutrients
- Internal kit controls for each assay plate
Reference Values
Reference intervals depend on each analyte and should be interpreted using WHO, BRINDA, or survey-validated cut-offs.
Turnaround Time
Results are available within 14 working days for up to 150 samples. For larger batches, please contact the laboratory team.
References
- Petunjuk Permohonan Pemeriksaan Contoh Uji (F.7.4.8)
- CDC, WHO, Nutrition International, UNICEF. Micronutrient Survey Manual. WHO, 2020.
For more information please contact:
lab@seameo-recfon.org
Hematology Analysis
Description
Hematology analysis provides essential information on red blood cells, white blood cells, and platelets to support the assessment of anemia, infection, inflammation, and overall health status. For field-based research, capillary hemoglobin is measured a portable photometric device suitable for community or survey settings. For laboratory-based testing, a complete blood count (CBC) is performed using the hematology analyzer, which provides automated, high-precision quantitative parameters for clinical and research applications. These complementary methods allow flexible hematological assessment in both field and laboratory environments, supporting population surveys, nutrition programs, and clinical research studies.
Useful For
- Screening and monitoring anemia
- Assessing hematological status in nutrition and health surveys
- Supporting diagnosis of infection, inflammation, or hematologic abnormalities
- Monitoring treatment or program interventions
Method
Field Hemoglobin Measurement (HemoCue Hb 201+)
- Photometric method using microcuvette technology
- Suitable for capillary (finger-prick) or venous blood
- Designed for field and community survey settings
Complete Blood Count (CBC) – Sysmex XP-100
- Automated impedance and non-cyanide hemoglobinometry
- 20 standard parameters, including RBC indices, WBC differential (3-part), and platelet count
- Internal quality control and automated calibration support
Sample Requirements
Sample types:
- Capillary whole blood (finger prick) – HemoCue Hb 201+
- Venous whole blood (EDTA) – Sysmex XP-100
Sample volume:
- HemoCue: 10–20 µL
- Sysmex XP-100: 1–2 mL EDTA whole blood
Patient preparation:
- No specific fasting required
- Ensure adequate hydration
- For capillary sampling: warm hands, clean skin, no lotion/powder
Sample Handling and Preparation
- EDTA tubes must be gently inverted (8–10 times) immediately after collection
- Avoid clotted or hemolyzed samples
- CBC samples should be analyzed within 6–8 hours at room temperature or within 24 hours if refrigerated (2–8 °C)
- HemoCue cuvettes must be protected from humidity and used before expiration
Quality Control (QC)
- Internal daily quality control for Sysmex XP-100 using commercial hematology controls
- Routine calibration according to manufacturer guidelines
- HemoCue quality verification using liquid controls
Reference Values
Reference ranges vary by age, sex, and population. Commonly reported parameters include:
- Hemoglobin (Hb)
- Hematocrit (Hct)
- Red cell indices (MCV, MCH, MCHC)
- White blood cell count and differential
- Platelet count and platelet indices
Turnaround Time
Field hemoglobin (HemoCue): Immediate (same day)
Laboratory CBC (Sysmex XP-100): 1–2 working days for routine batches
For large surveys, turnaround time can be adjusted based on sample volume—please contact the laboratory team.
References
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011
- Manufacturer’s documentation: HemoCue® Hb 201+ System
- Sysmex Corporation. Sysmex XP-100 Operator’s Manual
For more information please contact:
lab@seameo-recfon.org
Visit Us
Jl. Utan Kayu Raya No.1A, RT.1/RW.8, Utan Kayu Utara, Kec. Matraman, Kota Jakarta Timur, Daerah Khusus Ibukota Jakarta 13120
Phone Us
+62-21 22116225
Email Us
information@seameo-recfon.org
